PEPTIDE RECEPTOR RADIONUCLIDE THERAPY (PRRT)
What is PRRT and how does it work?
Peptide Receptor Radionuclide Therapy (PRRT) uses a radiolabelled somatostatin analog that binds to receptors on tumour cells, combined with radioactive compounds, including 111Indium, 68Galiium, 90Yttrium, 177Lutetium, to deliver the killing effect of the radioactivity directly to the cell, avoiding destruction of healthy cells often found with external beam radiation.
Lutetium Lu 177 dotatate (Lutathera)
| LUTATHERA® (lutetium Lu 177 dotatate) PRODUCT MONOGRAPH ENGLISH PRODUCT MONOGRAPH FRENCH |
|
|---|---|
| Brand Name | Lutathera |
| Generic Name | Lutetium Lu 177 dotatate |
| Strength | 370 MBq/mL |
| Indication | Lutathera® (lutetium (177Lu) oxodotreotide) was approved by Health Canada in January 2019, for the treatment of unresectable (not removable by surgery) or metastatic, well-differentiated, somatostatin receptor-positive (expressing the somatostatin receptor) gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in adults with progressive disease. |
| Tumour Type | Gastrointestinal |
| Contraindications |
|
pCODR Recommendation
Final Recommendation was issued on August 01, 2019 – Reimburse with clinical criteria and/or conditions* for treatment of adult patients with somatostatin receptor positive ( SSR+) midgut neuroendocrine tumours (NETs ) whose disease has progressed on a somatostatin analogue and is unresectable (see conditions )
INESSS Recommendation
Recommendation was issued February 2019 – Funded as a Médicament d’exception for neuroendocrine tumours in the digestif tract or pancreatic well differentiated, advanced disease, non resectable or metastatic expressing the somatostatin receptor in adults. ( see conditions )
ACCESS TO LUTATHERA IN CANADA
Cross Cancer Institute
Contact: 780-577-8080 (NET group administrative support)
Dr. Stella Koumna
Arthur J.E. Child Comprehensive Cancer Centre
Contact: 1-844-465-6330
Dr. Alda Aleksi and Dr. Denise Chan
BC Cancer will reimburse LUTATHERA for the approved indication.
The BC Cancer Benefit Drug List, Updated May 1, 2024
Peptide Receptor Radionuclide Therapy (PRRT) using 177Lu-Dotatate (LUTATHERA) for Treatment in Patients with Somatostatin Receptor Positive Neuroendocrine Tumours of the midgut or pancreas.
Pancreatic NET:
lutetium Lu 177 dotatate (LUTATHERA®), for the treatment of adult patients:
-
- With unresectable or metastatic, well-differentiated, somatostatin-receptor(SSR)-positive pancreatic neuroendocrine tumours (pNETs) AND
- Whose disease has progressed after treatment with a somatostatin analogue (SSA) OR
- With a contraindication or intolerance to SSAs
Treatment with LUTATHERA® should continue until disease progression, unacceptable toxicity, or up to a maximum of 4 infusions administered q8weekly (can be extended to up to 16 weeks in the event of adverse events requiring recovery).
Midgut:
lutetium Lu 177 dotatate (LUTATHERA®), for the treatment of adult patients:
-
- With somatostatin receptor-positive (SSR+), midgut* neuroendocrine tumours (NETs) AND
- Whose disease has progressed on a somatostatin analogue and is unresectable AND
- With good performance status.
*midgut defined as jejunoileum and proximal colon in the NETTER-1 trial
Treatment with LUTATHERA® should continue until disease progression, unacceptable toxicity, or up to a maximum of 4 infusions administered q8weekly (can be extended)
As of August 1, 2020, patients with midgut, GEP-NETs will now be eligible for treatment with LUTATHERA® [lutetium (177Lu) oxodotreotide]. This listing covers adult patients with somatostatin receptor-positive (SSR+) midgut NETs whose disease has progressed on a somatostatin analogue and is unresectable (not removable by surgery).
QEII Health Sciences Centre
1276 South Park, Halifax, NS
The Ontario funding criteria covers the treatment of adult patients with somatostatin receptor-positive (SSR+) midgut NETs whose disease has progressed on a somatostatin analogue and is unresectable (not treatable by surgery). Eligible patients include those with progressed SSR+ midgut (defined as jejunoileum and proximal in the NETTER-1 trial) NETs and good performance status.
Please see the press release:
ENGLISH – can be viewed at http://www.newswire.ca/en/releases/archive/October2020/08/c9204.html
FRENCH – can be viewed at http://www.newswire.ca/fr/releases/archive/October2020/08/c5664.html
London Health Sciences Centre
800 Commissioners Rd, London, ON
Dr. David Laidley, Fax: 519-667-6734 (NET patient Oncologist can make a faxed referral)
Sunnybrook Health Sciences Centre
3075 Bayview Avenue, Toronto, ON
Dr. Sten Myrehaug, (NET patient Oncologist can make a referral)
Princess Margaret Hospital
610 University Ave, Toronto, ON
Dr. Rebecca Wong
As of September 30, 2020, the Québec Ministry of Health and Social Services has listed Lutathera on the Liste de Médicaments – Établissements. Lutathera will be reimbursed for GEP-NETs patients with advanced disease that is metastatic or not removable by surgery and has progressed despite standard somatostatin analogue therapy and whose ECOG (Eastern Clinical Oncology Group) score is 0 to 2. Please refer to the following link for the formulary listing: https://www.ramq.gouv.qc.ca/sites/default/files/documents/liste-med-etab-2020-09-30-fr.pdf
Please see the press release:
ENGLISH –can be viewed at http://www.newswire.ca/en/releases/archive/October2020/01/c7837.html
FRENCH –can be viewed at http://www.newswire.ca/fr/releases/archive/October2020/01/c9369.html
CIUSSS Centre-Ouest de Montreal (JGH)
3755 Chemin cote Ste-Catherine, Montreal, QC
CHUM-Centre hosp. de l’Uni. de Montreal
1115 Rue Sanguinet, Montreal, QC
McGill University Health Centre (MUHC)
1001 Decarie, Montreal, QC
CHU de Quebec Universite (CHUQ)
11 cote du Palais, Quebec City, QC
Saskatchewan funding criteria:
Unresectable, well differentiated midgut* neuroendocrine tumors (NET) in patients who have experienced radiographic disease progression during somatostatin analog therapy (e.g., octreotide, lanreotide)
Lutathera (177Lu-dotatate) may be given for a maximum of 4 doses or until disease progression or unacceptable toxicity.
NEW LUTATHERA WEBSITE LAUNCHED
Advanced Accelerator Applications Canada, Inc. has launched an English and French website for patients and healthcare professionals to provide information on treatment with Lutathera.
Please visit: www.lutathera.ca
To get the Lutathera DIN, click on the attached Link: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
Enter Lutathera under product name.
NETTER-1 Study
NETTER-1 Study – Unmet need for long-term effective treatment of inoperable, gastro-entero-pancreatic neuroendocrine tumours (GEPNET).
This first Phase III trial compares 177Lu-DOTATATE with Octreotide (Sandostatin® LAR Depot) in patients with inoperable, progressive, somatostatin receptor positive midgut carcinoid tumours. 230 patients are divided into two groups of 115 each. The primary objective is to compare Progression Free Survival (PFS) between the groups. Secondary objectives are to compare Objective Response Rate, Overall Survival (OS), Time to Tumour Progression between groups, and to assess safety, tolerability and quality of life. 35 European and 15 U.S. sites are involved.
See trial details at: NETTER-1 STUDY
FACTS ABOUT PRRT
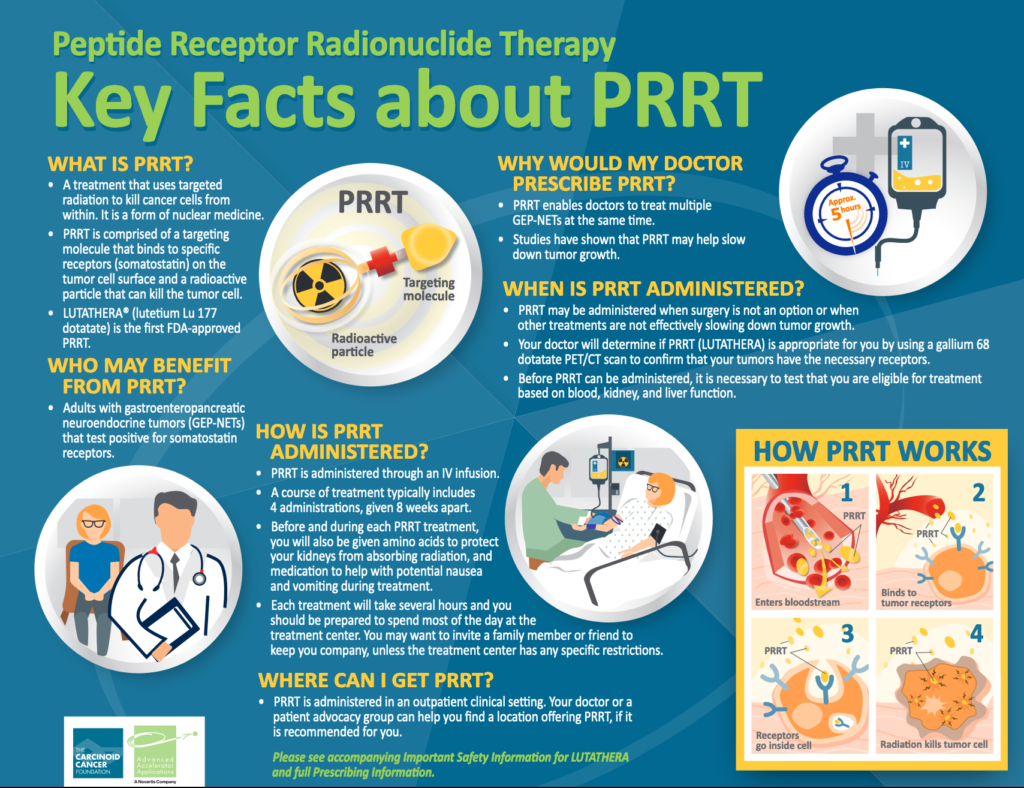
PRRT Trials In Canada
| Personalized PRRT of Neuroendocrine Tumors | Drug: 177Lu Octreotate | CHU de Québec – Université Laval Quebec City, Quebec, Canada |
| An Open Label Registry Study of Lutetium-177 (DOTA0, TYR3) Octreotate (Lu-DOTA-TATE) Treatment in Patients With Somatostatin Receptor Positive Tumours | Other: Lu-DOTA-TATE | London Health Sciences Centre London, Ontario, Canada |
| A Trial to Assess the Safety and Effectiveness of Lutetium-177 Octreotate Therapy in Neuroendocrine Tumours | Drug: [177]Lu-DOTA-TATE | Cross Cancer Institute Edmonton, Alberta, Canada |
| Lu-DOTATATE Treatment in Patients With 68Ga-DOTATATE Somatostatin Receptor Positive Neuroendocrine Tumors | Drug: Lutetium-177 Octreotate | Juravinski Cancer Centre Hamilton, Ontario, CanadaLondon Health Sciences Centre London, Ontario, CanadaPrincess Margaret Cancer Centre Toronto, Ontario, CanadaSunnybrook Odette Cancer Center Toronto, Ontario, Canada |
Advanced Accelerator Applications, AAA, has a new website regarding PRRT with Lutathera for both patients and providers: https://lutathera.com/. This website is intended for US residents only, there is a separate link for Non-US residents. There is also a separate section within the site for Healthcare Providers.
Peptide Receptor RadioTherapy for Neuroendocrine Tumours
Third Theranostic World Congress – Patient Edition
For full videos from the 3rd Theranostic World Congress that might be of more interest to NET Patients

